Abstract
Introduction
In utero hematopoietic cell transplantation (IUHCT) of allogenic bone marrow mononuclear cells (BM MNC) results in mixed hematopoietic chimerism in the murine model. In contrast, highly enriched HSCs (LSKs: Lin- c-Kit+ Sca-1+ cells) do not engraft after allogeneic IUHCT. Tracking studies in the first 72 hours after IUHCT demonstrate relatively large numbers of donor cells in the fetal thymus after IUHCT of BM MNCs, but not after IUHCT of LSK cells. This suggests that a sub-population of BM MNCs are capable of homing to the thymus within a window of opportunity for tolerance induction and that this population is lost, with enrichment of HSC.
The aim of this study is to identify the specific cell population within BM MNCs that is responsible for tolerance induction. To achieve this goal, we developed a practical model for determination of prenatal tolerance induction and tested subpopulations of BM MNC for their ability to induce tolerance.
Methods
Donor BM MNCs and subpopulations were isolated from 6-week old C57BL/6TgN(act-EGFP)OsbY01) (B6GFP) mice. Lineage (CD3, B220, CD11b, Gr-1) or CD3 depletion of donor cells was performed using magnetic sorting (Auto-MACS Pro Separator) and anti-Biotin microbeads.
To isolate the Lin- subpopulations, anti-biotin antibody was used to exclude Lin+ cells, and then the remaining cells were stained for c-Kit and Sca-1 markers.Donor subpopulations were generated by sorting using the FACSAria.
IUHCT was performed at E14 in Balb/c fetuses via vitelline vein injection. Post-natal injections were performed at P0 through the facial vein. Donor cell engraftment was assessed in peripheral blood at 4 and 12 weeks of age by flow cytometry (% GFP+ cells within CD45+ population).
Thymi and livers of E16 fetuses were harvested and GFP cells were detected using a fluorescence stereomicroscope.
Statistical analysis was performed using 1-way ANOVA with Bonferroni post-hoc tests. Results are expressed as mean±SEM. Experimental protocols were approved by the Institutional Animal Care and Use Committee at The Children's Hospital of Philadelphia.
Results
To develop the model for tolerance assessment we determined that injection of 10^7 CD3 depleted BM MNCs into Balb/c pups at P0 resulted in no engraftment. In contrast, IUHCT at E14 of 10^7 BM MNCs resulted in chimerism (13.2±2.01% chimerism) at 4 weeks with associated donor specific tolerance. Finally, P0 injection of 10^7 CD3 depleted BM MNCs into in mice that had received E14 injections of 10^7 BM MNCs resulted in engraftment with enhancement of chimerism at 4 weeks (38.02±3.67% in E14 plus P0 group vs 0.008±0.005 in P0 only group, p=0.0001).
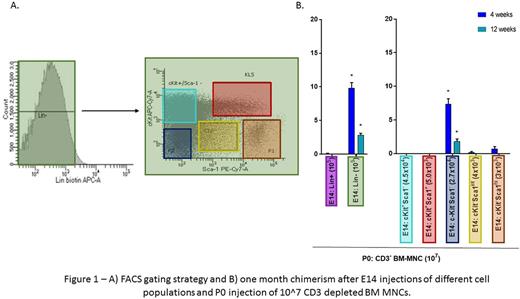
After establishing the model to test tolerance, we performed IUHCT of subpopulations of cells derived from BM MNCs, using the dose of each subpopulation found in 10^7 BM MNCs, to test their potential for tolerance induction. Mice that underwent IUHCT of 10^5 Lin- cells prior to the P0 injection of 10^7 CD3depleted BM MNCs had higher chimerism at 4 weeks than mice that received E14 injections of 10^5 Lin+ cells (9.843±0.79% vs 0.088±0.056%, p=0.0001).
Thymuses were harvested from E16 fetuses that were injected at E14 with different subsets of the Lin- population (c-Kit+ Sca-1+, c-Kit- Sca-1+, c-Kit+ Sca-1-, c-Kit- Sca-1int, and c-Kit- Sca-1- cells). The only thymuses to show GFP cells at E16 were those that had received E14 Lin- c-Kit- Sca-1- cells. Mice that received Lin- c-Kit- Sca-1- cells at E14 prior to P0 injections showed chimerism at 4 weeks when compared to those that had received Lin- c-Kit+ Sca-1-, Lin- c-Kit+ Sca-1+, Lin- c-Kit- Sca-1int, or Lin- c-Kit- Sca-1hi cells at E14, in which chimerism was absent (6.755±0.95% vs 0.0%; p=0.02.)
Conclusion
We have demonstrated that it is the Lin- c-Kit- Sca-1- subpopulation of BM MNCs that induces tolerance and prevents rejection of subsequent injections from the same donor. We have also shown that only the Lin- c-Kit- Sca-1- population migrates to the thymus in time for the induction of central tolerance. Furthermore, mouse fetuses that were given Lin- c-Kit- Sca-1- intravascularly at E14 were able to become chimeric with a postnatal CD3 depleted MNCs transplantation without immunosuppression or myeloablation. These findings suggest that a c-Kit- Sca-1- progenitor is responsible for tolerance induction, likely via common dendritic progenitors that home to the thymus.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal